WORLD FEDERATION FOR CULTURE COLLECTIONS
GUIDELINES
FOR THE ESTABLISHMENT AND OPERATION
OF
COLLECTIONS OF CULTURES
OF
MICROORGANISMS
3rd Edition, February 2010
Revised by the WFCC Executive Board
BACKGROUND
The World Federation for Culture Collections (WFCC) is a COMCOF (Committees, Commissions and Federations) of the International Union of Microbiological Societies (IUMS) and a scientific member of the International Union of Biological Sciences (IUBS). It’s key objective is the promotion and development of collections of cultures of microorganisms and cultured cells. Retention and support of existing col ections, as well as assistance and advice to help new col ections become established remain key activities. The members of WFCC constitute a unique global network for ex situ preservation of microbial diversity which underpins life on earth. This is particularly pertinent in 2010, the International Year of Biodiversity. The WFCC has an on-going concern with all aspects of culture collection activity and, in particular, with the encouragement of new initiatives and improvement of the quality standards of scientific services provided to the international user community.
The increasing demands on culture col ections for authenticated, reliable biological material and associated information have paralleled the growth of biotechnology. More recently, the Organisation for Economic Co-operation and Development (OECD) have recognised the importance of taking culture collections to a higher level of quality and delivery to underpin biotechnology. One key element of this development is the introduction of best practice (OECD, 2007), for which the WFCC guidelines laid the foundation. These guidelines have been updated to include recent developments and changes to provide basic quality management guidance for culture col ections. The OECD Best Practice Guidelines for Biological Resource Centres (OECD, 2007) set the standard for quality management and also covers biosecurity, building capacity, preservation of biological esources and data management. The WFCC guidance provides an excellent first step towards the implementation of the OECD Best Practice. It is anticipated that many member collections will be able to implement this guidance in full immediately but it is expected that each agrees to implement it in a reasonable time frame.
It is hoped that these Guidelines prove valuable and encouraging. The WFCC wishes to emphasise that high standards of scientific service can be achieved in laboratories with modest resources and that sophisticated equipment is not a prerequisite for good microbiological practice; the principles listed in the Guidelines must be applied to any culture collection regardless of size or economic standing.
EXECUTIVE SUMMARY
These Guidelines are prepared by the WFCC to provide a framework for the establishment, operation and long-term support of microbiological and cell resource centres as a fundamental part of the scientific infrastructure.
The Guidelines describe:
· The aims of culture collections
· The services they provide to the international scientific community in terms of
resources, information and specialist skils
· The long-term support needed to enable them to provide these professional
services, including:
o Appropriate operational facilities
o The staffing levels to allow operation at a high standard
o The training level of staff with research expertise related to the aims of the
collection
· The contributions made by collections to the research knowledge base in terms of
taxonomic studies, preservation, growth and handling procedures and other linked
areas
· The capability of collections to meet all relevant national and international
regulations concerning the control, transportation and health and safety aspects of
resource handling and distribution
· The need to provide support and training in capacity building on a global basis;
· The need for international collaboration to enhance the value and quality of
biological resources
· References and web site links
The guidance demands compliance with national legislation, rules and regulations.
CONTENTS
1. Introduction
2. Organisation
3. Funding
4. Objectives
5. Holdings
6. Staff
7. Preservation
8. Culture Authentication
9. Culture Supply
10. Other Services
11. Documentation
12. Catalogues
13. Research
14. Training
15. Safety and Security
16. National and International Collaboration
17. Compliance with Legislation
Selected Bibliography and Web Sites
Useful Addresses
INTRODUCTION
1.1 The ever decreasing investment in traditional taxonomy, the increasing demand for a molecular approach, the continued depletion of natural resources and concerns over biosecurity and climate change brings a heightened awareness of the value of collections of microorganisms. Conservation of genetic resources and biodiversity provides the essential underpinning for emerging biotechnologically based eco-efficient products and industries in both the developed and the developing world (OECD, 2001); an essential element in the development of a knowledge-based bioeconomy (OECD, 2009).
1.2 Many countries and individual institutions therefore have established or are establishing publicly supported culture collections of microorganisms for the first time, either to provide services to their country or region or in support of their own research programmes.
1.3 The first edition of these guidelines in 1980 was the first attempt to develop guidelines for culture col ections. Since then, numerous guidance documents have been developed (see Safety and Standards websites below) these, and international standards are being applied to the operations of collections today.
1.4 The objective of these Guidelines is to provide assistance to those collections of
microorganisms offering services outside their own institution (service collections), but it is
anticipated that many of the guidelines will be more generally applicable to in-house or
research collections. Guidance such as the CABRI guidelines (http://cabri.org) and the
OECD Best Practice for Biological Resource Centres are designed for public service
collections and are the next level of guidance, which require extensive investment to
implement.
1.5 WFCC expects that, wherever possible, service collections will adopt the Guidelines enumerated here. Membership of the WFCC includes the obligation to implement these standards to guarantee consistent and sustainable quality of authentic materials and information.
ORGANISATION
2.1 The parent organisation, or board, under which a culture collection is established should be fully aware of and accept the responsibilities inherent in maintaining a public service to appropriate standards. Commitment to the maintenance of the col ection and its services in the long-term should therefore be included in the strategic plans or objectives of the parent organisation as appropriate. In the case of existing collections, where this responsibility is not explicit, this aspect should be clarified with the Director of the parent institute, its Scientific Council, senior university officials, Governing Board, or other such authorities as may be appropriate.
FUNDING
3.1 Administration and funding arrangements for collections require a long-term commitment
from the parent organisation. Support solely in the form of short-term contracts or without any
allocation of core funding is inappropriate for service collections aiming to provide long-term
storage and supply services. Even the establishment of small in-house collections requires an
ongoing source of direct, or indirect, financial support from a parent body.
3.2 It is important to consider the level of funding, both now, and likely to be forthcoming on an on-going basis. This must be adequate to provide the range of services being planned and at a standard that users would expect. If secure resources are limited, in general it is preferable to restrict the primary objectives of the col ection to those which it has a strong probability of maintaining in the long-term.
OBJECTIVES
4.1 Collections require a clearly summarised general statement of their long-term objectives relating to the scope of their holdings and to the range of outside services that are envisaged.
4.2 In addition, it is often helpful for a collection to have more specific short-term objectives
relating to the coming 1, 3 or 5 -year period. These can usefully include the numbers and
groups of strains which it is planned to acquire in that time frame and schedules for installing
new facilities and services.
4.3 Where possible a mission statement in accordance with 4.1 and 4.2 should be prepared which is sufficiently short to reproduce in promotional and other material disseminated.
HOLDINGS
5.1 The scope of material and numbers of strains to be held requires careful consideration and merits discussion with the parent organisation and any funding bodies concerned when the col ection is being established, as this wil have long-term financial implications.
5.2 In addition to decisions on the groups of microorganisms to be maintained, and the numbers it is envisaged as being retained in the long-term, it is also necessary to have a clearly defined accessions policy on which new strains are to be taken into the collection. If this is not decided and many unsolicited strains are accepted uncritical y without due regard to the col ection's objectives, storage capacities, personnel and financial resources can soon become overstretched; at the same time, the range should not be so strictly defined as to limit the effectiveness of the services provided to the users. Collaboration with other collections to provide broader coverage is essential, networking activities to enable co-ordinated accession policy must be considered, whether at a regional, national or global level (see paragraph 16.1)
5.3 If strains are maintained that are potentially pathogenic to man, animals or plants, or
produce toxic or hallucinogenic compounds, those holdings should be clearly label ed and
kept secure; adherence to any safety regulations in force is mandatory. National legislation
impacts on this and many countries require permits or licences to hold, work with and
distribute such organisms (see EBRCN legislation document on WFCC website).
5.4 Collections vary substantial y in scope with regard to the groups of microorganisms held,
geographical emphasis, and user-group orientation. It is beneficial to stress at an early stage
areas in which the holdings are planned to become particularly rich as this will be of the
utmost value to both potential depositors of strains and those wishing to acquire strains or
requiring other services.
5.5 In considering which strains to maintain, it is economically prudent to aim at complementing rather than duplicating those already available through other service collections. While it may be desirable for collections to include some authenticated internationally recognised reference strains, the WFCC wishes to discourage the unnecessary use of scarce resources. Wherever possible, new collections of microorganisms being established should collectively enrich the world's available genetic resources rather than duplicate those already existing.
5.6 In determining which strengths a new collection should have with respect to its holdings,
particular attention should be paid to those already present in that particular country or region
as well as those providing international services. Information as to which collections already
exist can be obtained from the WFCC World Data Centre for Microorganisms (WDCM) –
online via the WFCC website. Some other specialist listings are also available (e.g. CABRI,
ECCO, JCM etc.).
STAFF
6.1 Culture collections are necessarily labour-intensive. When determining the numbers of full
and part-time positions required it is important to consider how time-consuming the routine
accessions, preservation, maintenance, and viability checking will become as the collection
approaches its target strain numbers. Staff levels need to be sufficient not only for the
incorporation and maintenance of cultures, but also to fulfil the anticipated level of culture
supply and other services the collection is to offer.
6.2 The effective curation and management of a culture collection is a demanding task. It requires knowledge not only of the organisms themselves, but also their growth and preservation requirements, properties and potential applications and the provision of customer services. The key staff member(s) recruited would be expected to have a higher degree in an appropriate field and some subsequent direct experience or special training in culture col ection curation skil s. In order to attract and retain sufficient calibre staff, arrangements for ongoing employment should be made. Too frequent staff turnover will jeopardize the maintenance of standards in the collection and hence the quality and effectiveness of the services provided.
6.3 Particular attention should be paid to the qualifications and experience of the persons in charge of the Col ection.
6.4 While it is not always practical to have on staff specialists concerned with, for example, the identification and authentication of al systematic groups covered, some basic taxonomic skil s are essential for quality control (see para 8.1). Where a need for specialist taxonomic support exists, especially if it relates to services such as identification being advertised, steps need to be taken to provide such expertise through collaborative arrangements within and(or) outside the collection's parent organisation. As such specialist assistance might be required at short-notice, it is preferable for such arrangements to be formal rather than informal.
PRESERVATION
7.1 Different microorganisms often require special preservation methods in order to ensure optimal viability, storage, and purity. For security, and in order to minimise the probability of strains being lost, each strain should, whenever practical, be maintained by at least two different procedures. At least one of these should be by freeze-drying (lyophilisation) or storage at ultra low temperature in liquid nitrogen or mechanical freezers maintaining temperatures of -140°C or lower cryopreservation); these are the best methods for minimising the risks of genetic change. In some cases, for example cell lines, where only freezing is available, duplicates should be stored in separate refrigerators with different electrical supplies. (See also para 7.3)
7.2 While considerable experience is now available on the optimal preservation methods for many groups of microorganisms, this is not so for all. Particular care is needed with genera and species hitherto not preserved in culture col ections when a greater range of procedures should be attempted or research carried out to determine optimal protocols (See para 14.2).
7.3 In order to minimise the risks to important genetic resources from fire, flooding, earthquakes, war or catastrophes, col ections should arrange to have duplicates of at least the most important and irreplaceable strains (and also of their associated documentation) securely housed in a different building or ideally at a separate site.
CULTURE AUTHENTICATION
8.1 Scientists ordering cultures from collections expect them to be correctly identified. If not,
there is a danger of users employing the wrong organism in their investigations which could prove time-wasting, expensive, and lead to invalid published results. The name applied to a
strain leads into other information relevant to that species including risk group, potential toxin
production, biosecurity risks and therefore it is of critical and prime importance that the name
assigned is correct. Moreover, without proper authentication noxious organisms could be
inadvertently supplied. This places a grave responsibility on col ections and demands
attention from the time the first cultures are received for preservation. WFCC member
collections have a responsibility to provide resources with accurate identities as reference
materials if they offer a public service and must make every effort to ensure that organisms
they supply are authentic.
8.2 When named cultures are received, the person making the original identification should be recorded. The Col ection should confirm the identification and check that it agrees with published descriptions of the species. Alternatively, the Collection should confirm that it has been checked by a competent specialist or by comparison with authorised molecular data or other profiles.
8.3 In the case of unidentified cultures received, the Collection should be wary of identifying material in groups for which it has no specialist taxonomist and it should endeavour to have material checked by specialists prior to incorporation. Such materials are to be treated with care and assumed to have a high level of risk until a risk assessment and/or the name of the organism has been established.
8.4 In the case of microorganisms which are recognisable from microscopic preparations or dried cultures (i.e. filamentous fungi, algae, protozoa), it is good practice to make such preparations when they are received for deposit, and/or establish molecular barcodes or other profiles (e.g. MALDI-TOF, fatty acids). This facilitates the checking of whether a strain recovered from the collection conforms to that originally deposited.
8.5 The first time (and at appropriate regular intervals afterward) cultures are recovered from the Col ection, during aintenance or routine re-preservation work, or when they are being dispatched, care should be taken to ensure they conform to the original deposit by carrying out appropriate tests, by comparative study (See para 8.4), or checking by a specialist.
8.6 The need to authenticate cultures must be borne in mind when staff are recruited, and arrangements for access to specialists have to be made (See para 6.4).
CULTURE SUPPLY
9.1 Collections should be able to distribute cultures listed as available which are requested. Arrangements for culture supply vary according to the financial basis and policies of the legal owners of the Col ection.
9.2 Cultures listed as available in catalogues by service collections should normally be provided without prejudice to those requesting them, subject to any import, quarantine or containment regulations that might apply and to normal credit control procedures where charges are required to be made. It is recognised that charging policies and differential rates for users in particular regions or for different purposes (for example teaching vs. industry) may have to be applied in accordance with the policy of the parent organisation or funding body.
9.3 In offering a culture supply service, consideration needs to be given to the provision of sufficient staff to satisfy the numbers of requests it is likely to receive in a timely manner. Cultures that cannot be dispatched for technical reasons within a reasonable time of receipt of an order with any necessary permits, should be indicated in the Catalogue.
9.4 Strains which are pathogenic or toxic to plants, animals or man often are subject to
regulations from health and(or) agriculture authorities. Scientists requesting strains may need to obtain permits to import material or to handle certain cultures. There are several elements
of legislation that impinge upon distribution of organisms:
· Quarantine – mainly plant (crop) and animal diseases
· Biosafety – restriction on biosafety level (risk group) or hazard level that can be
handled by the recipient
· Biosecurity – control legislation on the movement of dangerous pathogens
· Intel ectual Property – for example, Patent Strains under the Budapest Treaty
often require certificate of release (see para 10.3)
Where cultures are being supplied to a person or institution not known to the Collection, guarantees should be obtained on the credentials of the person concerned and other facilities of the institution before dispatching cultures.
9.5 Collections should maintain detailed records of recipients of cultures showing the material sent (with strain and batch numbers where appropriate), method and date of shipment, and name and address of the person to whom sent. In the case of unsatisfactory results or if it is necessary to supply subsequent information recipients can then be notified. It is recommended that collections utilise Material Transfer Agreements (MTAs) to ensure the recipient is aware of any of the terms and conditions of access. Example minimum text for such MTAs can be found in the ECCO MTA http://www.eccosite.org). Complementary information is provided by MOSAICC (see Micro-organisms culture collections, Micro-organisms Sustainable Use and Access Regulation International Code of Conduct (MOSAICC) at http://www.cbd.int/abs/instruments/).
9.6 In dispatching cultures, attention needs to be given to pertinent postal and shipping regulations regarding packaging and labelling, see Selected Bibliography and para 9.5 9.7 The WFCC require al member collections and recommends to all others that TYPE strains must be made available without restriction to the scientific community.
OTHER SERVICES
10.1 Service culture collections may be wel placed to provide a variety of support services to the scientific and industrial community worldwide or in the region they serve. If such extension services are contemplated, they need to be carefully planned as they frequently require additional expertise and facilities.
10.2 If identification services are to be offered it should be considered whether appropriately
trained personnel are available to undertake this demanding task, either in the collection or in
an associated institution. Major problems can arise as a consequence of misidentifications
(See paras 6.4, 8.1).
10.3 Where international patent depositary facilities are to be provided, these should be
operated according to the procedures laid down in the Budapest Treaty on the International
Recognition of the Deposit of Microorganisms for the Purposes of Patent Procedure
(Regulations, 1977; Guide to the Deposit of Microorganisms under the Budapest Treaty, 1988
[both published by World Intellectual Property Organization (WIPO), Geneva]). In such cases
the col ection would have to qualify under WIPO rules to satisfy the stringent regulations
required to become accepted as an International Depositary Authority (IDA). A code of conduct for International Depository Authorities is available at http://bccm.belspo.be/tbu/ida/index.php.
10.4 If consultancy, advisory or investigation services are to be offered, attention must be given to the provision of appropriate facilities and properly trained personnel (See para 8. 1).
DOCUMENTATION
11.1 Records need to be kept for each strain held and should, at least, include the following categories of information:
· Place
· Substrate or host
· Date of isolation
· Name of person isolating the strain
· Depositor (or other source of the strain, such as from another Col ection)
· Name of the person identifying the strain
· Preservation procedures used
· Optimal growth media and temperatures
· Data on biochemical or other characteristics
· Regulatory conditions applying (relating for example to quarantine, containment
levels and patent status)
WDCM provides for an efficient coding of the strains by defining a collection acronym and WFCC number which allows each culture col ection to give a Global y Unique Identifiers (GUID) to each strain of its holding, combining their acronym with their own internal numbering. The pioneering work of WDCM enables an appropriate recording and management of the documentation related to the strains. Collections should use this system to be part of the WDCM network and be connected to the international scientific community.
11.2 Whenever resources permit, the records should be computerised. Collections are
encouraged to adopt a field structure and field definitions which will enable the data to be
integrated into the international and major regional schemes now in operation [e.g. Microbial
Information Network Europe (MINE), CABRI Guidelines, OECD Best Practice]. Several
compatible programmes exist and the WFCC, WFCC World Data Centre for Microorganisms
(WDCM), and CABRI can provide helpful information and suggestions on appropriate levels
of management of this information (see Bibliography). Even if data exchange is not being
planned in the short-term, it is wasteful of resources to develop independent systems that
already exist.
11.3 For security, duplicate computer files or photocopies of records should be kept separately, perhaps deposited with duplicate strains (See para 7.3).
11.4 Where records are computerised, several of the Collection's staff should be familiar with the operation of the system in order to provide cover during periods of absence.
CATALOGUES
12. Printed or on-line catalogues of the strains available for distribution should be produced or updated at regular intervals. While annual printed catalogues are rarely justified, gaps of five or more years would be too great to be useful. On-line catalogues should be updated more frequently. Cultures with restricted distribution should be clearly marked. Cultures which, for any reason, are not available for distribution should not be listed in catalogues or publicly accessible databases.
RESEARCH
13.1 Research programmes should – when possible - be a part of every Collection's activity. It not only helps attract staff of high calibre, but can make important contributions to knowledge of the morphology, taxonomy, physiology, biochemistry and genetics of the groups of organisms maintained. Research activities also ensure that staff keep abreast of current developments and are aware of the needs of the user community.
13.2 Collections are also wel -placed to develop screening procedures for particular organisms, preservation protocols for strains difficult to preserve by routine procedures and optimal cultural media and conditions for growth.
TRAINING
14.1 While Col ection staff require appropriate training themselves, once they have become skil ed they are wel -placed to train others in techniques relating to culture preservation, growth, and identification.
14.2 If training is to be provided, it is important to ensure that adequate provision is made for teaching facilities and supervision.
14.3 WFCC provides training often associated with its International Conference for Culture Collections (ICCC) but it also provides ad hoc training courses and has a work programme on capacity building. Additionally, many culture collections offer individual training on different issues.
SAFETY AND SECURITY
15.1 Safety aspects of all operations carried out in the Col ection include biosafety, chemical and physical safety etc and need to be carefully scrutinised with respect not only to national health and safety regulations, but also with regard to good laboratory practice. Risk assessments must be carried out before cultures are brought into the collection and specific procedures are applied. Adequate controls must be implemented to manage risk, not just to collection workers, but to all who may come into contact with cultures, products and services provided including the complete transportation chain.
15.2 Particular attention needs to be given to the containment and biosecurity aspects of
strains which are potential y harmful to man, animals or crops. WFCC requires member
collections to implement best practice on all safety and security aspects according to the
requirements and holdings of individual culture collections. In addition, increased levels of
security are an important consideration when a collection accepts secure, safe or patent
deposits where a col ection has additional client and legal obligations to satisfy.
15.3 Facilities wil be required for the safe opening of packages containing new deposits or material for identification which could contain harmful organisms. Al steps involved in accessioning new materials shall consider biosafety and biosecurity and clear responsibilities shal be laid down.
15.4 See section 17 on compliance with al aspects of legislation that are most relevant for culture col ections
NATIONAL AND INTERNATIONAL COLLABORATION
16.1 Many countries have formal or informal associations or federations of the collections within them. These provide excel ent opportunities for exchange of information and discussions of mutual problems and collections should be encouraged to support them.
16.2 Similarly, the establishment of formal or informal links with any regional groups active in adjacent countries should be encouraged. Examples of such links are the European Culture Collections' Organisation (ECCO) and the Microbial Resource Centres (MIRCEN) network.
16.3 In order to make their holdings widely known, collections are encouraged to register with the WFCC World Data Centre for Microorganisms (WDCM). It is also recommended that international standards for data exchange and interoperability are adopted to facilitate international communication and data exchange.
16.4 Collections and individual senior staff within collections may join the World Federation for
Culture Collections (WFCC). This has work programmes concerned with education, patents,
implementation of legislation, endangered collections and standards which all provide
information that may be of assistance to new and established collections. The WFCC holds a
major international congress every three years which provides a unique forum for the
consideration of all aspects of the activity of culture collections. A Newsletter is produced and
training schemes and courses are operated. Col ection staff should be encouraged to actively
participate in the affairs of the WFCC.
COMPLIANCE WITH LEGISLATION
17.1 Operations of culture collections must be carried out safely and compliant with the various legislation and regulations that control these matters. Moreover the legislation is subject to changes, which are not always directly communicated to the interested parties. The WFCC through its Newsletter and website endeavours to keep its membership and users informed. In the process of isolation, handling, storage and distribution of microorganisms and cell cultures there are many stages where compliance with the law, regulations or international conventions is required. A culture col ection should comply with:
Health and Safety requirements
Classification of Micoorganisms on the Basis of Hazard
Quarantine regulations
Ownership of Intel ectual Property Rights (IPR)
Convention on Biological Diversity
Safety information provided to the recipient of microorganisms
Regulations governing shipping of cultures
Control of Distribution of Dangerous Organisms
Budapest Treaty (for patent deposits)
Health and Safety
17.2 The institutions’ director/senior management is responsible for the implementation of
all relevant national regulations in the context of occupational health. A structure for
verifying this must be set up. The importance of a laboratory’s health and safety
procedures stretches beyond the laboratory to include all those who may come in contact
with substances and products from that laboratory. A risk assessment of handling and
supply of organisms is required and should include an assessment of all hazards involved,
not just infection, but also al others amongst which are, the production of toxic metabolites
and the ability to cause al ergic reactions. Organisms that produce volatile toxins or
aerosols of spores or cells present a greater risk. It is the responsibility of the scientist or
curator to provide such assessment data where known to a recipient of a culture to ensure
its safe handling and containment.
Regulatory control of microbiology
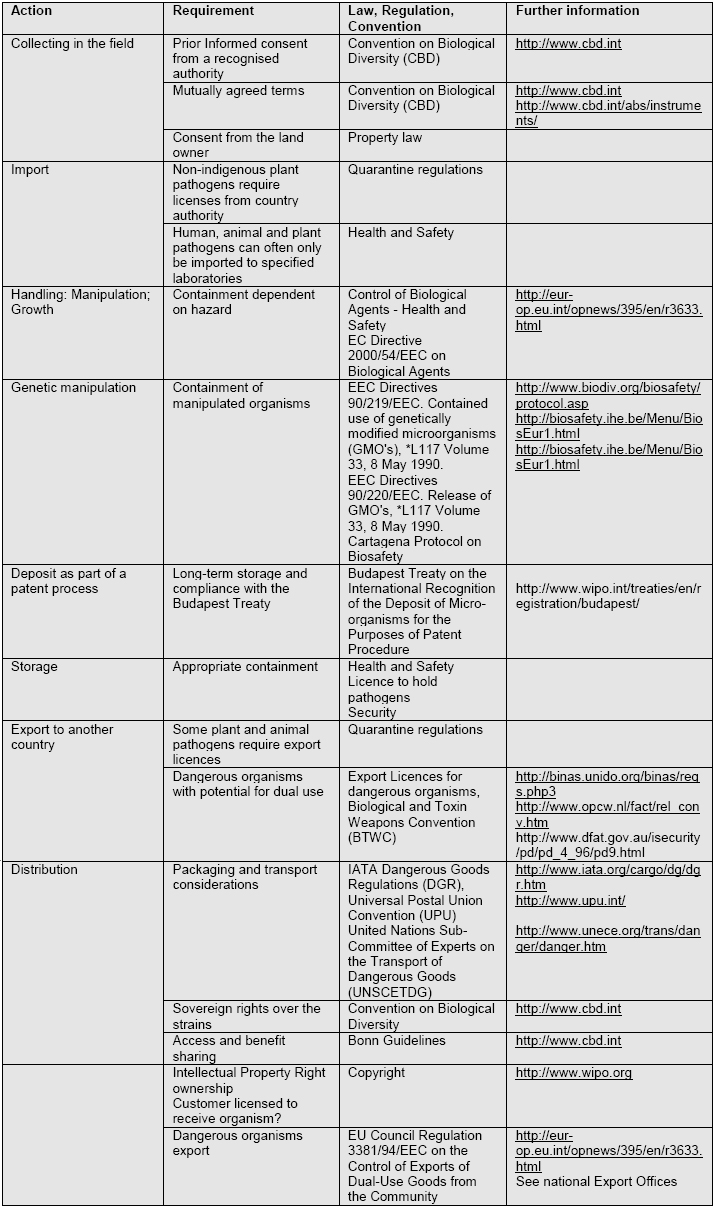
Classification of Microorganisms on the Basis of Hazard
17.3 Various classification systems exist which include the definitions for classification by the
World Health Organisation (WHO); Microorganisms are normally classified on their potential
to cause disease, their human pathogenicity, into four risk groups:
Risk Group 1 A biological agent that is most unlikely to cause human disease.
Risk Group 2 A biological agent that may cause human disease and which might be a hazard
to laboratory workers but is unlikely to spread in the community. Laboratory
exposure rarely produces infection and effective prophylaxis or treatment is
available.
Risk Group 3 A biological agent that may cause severe human disease and present a serious
hazard to laboratory workers. It may present a risk of spread in the community
but there is usual y effective prophylaxis or treatment.
Risk Group 4 A biological agent that causes severe human disease and is a serious hazard to
laboratory workers. It may present a high risk of spread in the community and
there is usual y no effective prophylaxis or treatment.
Classification of animal and plant pathogens, their handling and distribution are covered by national and regional legislation.
Quarantine Regulations
17.4 Clients who wish to obtain cultures of non-indigenous pathogens may first have to obtain
a permit to import, handle and store from the appropriate Government Department. Under the
terms of such a licence the shipper is required to see a copy of the Ministry permit before
such strains can be supplied.
Rights to further distribute
17.5 On deposit of biological materials culture collections must ascertain terms and conditions
of further distribution, for example, Intel ectual Property rights or from Prior Informed Consent
granted under the Convention on Biological Diversity.
Convention on Biological Diversity
17.6 The WFCC endorses the principles of the Convention on Biological Diversity and
requires biological materials to be received and supplied within the spirit of the CBD. First and
foremost the WFCC requires its members to follow its national legislation, rules or regulations,
which take precedence. The requirements laid down by countries of origin must be honoured.
Transfer of materials should be accompanied by material transfer agreements or other forms
of conditions of supply informing recipients of any terms and conditions that apply.
Safety Information provided to the Recipient of Microorganisms
17.7 It is recommended that a safety data sheet be despatched with an organism indicating
which hazard group it belongs to and what containment and disposal procedures are
necessary. A safety data sheet accompanying a microorganism should include:
· The hazard group of the organism being despatched
· A definition of the hazards and assessment of the risks involved in handling the
organism
· Requirements for the safe handling and disposal of the organism
- Containment level
- Transport
- Disposal
- Procedures in case of spillage
Regulations governing Shipping of Cultures
17.8 The IATA Dangerous Goods Regulations (DGR) require that shippers of microorganisms
of Risk Groups 2, 3 or 4 must be trained by IATA certified and approved instructors (every two
years) if cultures are sent by air transport. Transport of highly pathogenic material classified
in Category A, UN 2814 or UN 2900 (see definition of this shipping Category and Table 3.6D,
DGR 2010), requires shippers declaration forms, which accompany the package in duplicate. Cultures of infectious substances meeting the definition of shipping Category B, UN 3373
(majority of the Risk Group 2 organisms), can be transported under deregulated conditions.
Different labels and packaging specification markings are used for organisms in transit by air,
dependent on the shipping Category. IATA DGR also requires that packaging used for the
transport of Risk Groups 2, 3 or 4 must meet defined standards of a UN combination package.
See Addendum II to the current DGR 51st Ed., 2010 and IATA homepage http://www.iata.org. Category A shipments require a Packing Instruction PI 602 packaging whereas for Category
B shipments PI 650 packaging are accepted. PI 650 also meets the requirements of UPU for
the transport of Risk group 1 organisms. Generally, there is no lesser packaging quality than
PI 650. The WFCC homepage offers information on packaging and shipping.
Control of Distribution of Dangerous Organisms
17.9 There is considerable concern over the transfer of selected infectious agents capable of
causing substantial harm to human health, animals or crops. There is potential for such
organisms to be passed to parties not equipped to handle them or to persons who may make
illegitimate use of them. A culture collection must have procedures to check the validity of
customers that wish to receive dangerous organisms that present a biosecurity risk and if in
doubt must not supply.
The WFCC fully supports the Biological and Toxin Weapons Convention of 1972 (BTWC). But,
it is not the policy of the WFCC to influence the range of bioresources maintained or to
interfere with research activities of member collections. National governments and authorities
are the enforcers of legislation, control lies with the country in which the col ection is based.
The WFCC urges its members to strictly fol ow all national and international legislation
concerning distribution of sensitive materials to third parties. Such materials shall be clearly
labelled and kept secure. Collections should maintain detailed records of recipients of cultures. The
requestors/recipients may need to obtain permits to import or to handle the cultures. In case
of trans-border supplies, written and signed guarantees should be obtained on the credentials
of the requesting person before despatch if other authorisation is not available. Material
transfer agreements before despatch might be an additional security. In the case of new
customers, the recipient’s institution and the person’s name shall be checked against
international lists in the context of bio-terrorism.
SELECTED BIBLIOGRAPHY
Crous, P.W. (2003) Adhering to good cultural practice (GCP). Mycological Research News
1378-1379.
Day, J.G. & Stacey, G. (2006) Cryopreservation and Freeze-drying Protocols, 2nd ed.:
Springer, ISBN 1597453625, 9781597453622
Gams, W & Hennebert, G L, Stalpers, J, Janssens, D, Schipper, M A A, Smith, J, Yarrow, D &
Hawksworth, D L (1988) Structuring strain data for storage and retrieval of
information on fungi and yeasts in MINE, the Microbial Information Network Europe.
Journal of General Microbiology 134: 1667-1689.
Lima, N. & Smith, D. (2003). Biological Resource Centres and the Use of Microbes:
Proceedings of European Culture Col ection Organisation XXII, 17-19 September
2003. Braga, Portugal: Micoteca da Universidade do Minnho. ISBN 972-97916-3-5.
pp422.
OECD (2001). Biological Resource Centres: Underpinning the future of life sciences and
biotechnology. OECD Publications, Paris, France. pp 66.
OECD (2007). Best Practice Guidelines for Biological Resource Centres (June 2007),http://www.oecd.org/document/36/0,3343,en_2649_34537_38777060_1_1_1_1,00.html
OECD Best Practice Guidelines on Biosecurity for BRCs In: Best Practice Guidelines for
Biological Resource Centres (June 2007), http://www.oecd.org/document/36/0,3343,en_2649_34537_38777060_1_1_1_1,00.html
OECD (2009). The Bioeconomy to 2030: designing a policy agenda. OECD Publications.
Ryan, M.J. & Smith, D. (2004) Fungal Genetic Resource Centres and the genomic chal enge. Mycol Res 108, 1351-1362.
Ryan, J.M., Jeffries, P. & Smith, D. (2001). Developing cryopreservation protocols to secure
fungal gene function. Cryoletters 22, 115-124.
Ryan, M.J., Smith, D. & Jeffries, P. (2000). A decision-based key to determine the most
appropriate protocol for the preservation of fungi. World Journal of Microbiology & Biotechnology 16, 183-186.
Ryan, M.J., Smith, D., Bridge, P.D., & Jeffries, P. (2003). The relationship between fungal
preservation method and secondary metabolite production in Metarhizium anisopliae
and Fusarium oxysporum. World Journal of Microbiology and Biotechnology 19, 839-
844.
Smith, D, & Rohde, C. (2007) Biological Resource Centres and compliance with the law. UK:
Society for Microbiology http://www.sgm.ac.uk/pubs/micro_today/pdf/0299brc.pdf
Smith, D & Ryan, M.J. (2008). The impact of OECD best practice on the validation of
cryopreservation techniques for microorganisms. Cryoletters 29, 63-72.
Smith, D., M.J. Ryan & J.G. Day. (eds) (2001). The UK National Culture Col ection Biological
Resource: Properties, maintenance and management. pp 382. UK National Culture
Collection, Egham.
Smith, D. & Ryan, M.J. & Stackebrandt, E. (2008) The ex situ conservation of
microorganisms: aiming at a certified quality management. In Biotechnology [Eds.
Horst W.Doel e, Edgar J.DaSilva], in Encyclopedia of Life Support Systems (EOLSS).
Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford, UK
[http://wwweolss.net]
Smith, D. & Ryan, M.J. (2004) Current status of fungal collections and their role in
biotechnology. In Handbook of Fungal Biotechnology 2nd edition. (Arora, D.K., ed),
527-538.Marcel Dekker, Inc. New York.
Note: Changes to the Code are also documented in the minutes of the ICSP and its Judicial
Commission, published in the International Journal of Systematic Bacteriology/International
Journal of Systematic and Evolutionary Microbiology.
BIBLIOGRAPHY ON COMPLIANCE WITH LEGISLATION
Anon (1994). Approved Code of Practice for Biological Agents 1994. Health and Safety
Executive. Sudbury: HSE Books.
European Commission Directive 95/44/EC of 26 July 1995 establishing the conditions under
which certain harmful organisms, plants, plant products and other objects listed in
Annexes I to V to Council Directive 77/93/EEC may be introduced into or moved
within the Community or certain protected zones thereof, for trial or scientific
purposes and work on varietal selections. Official Journal No. L 184, 03.08.1995, p.
34
European Commission Directive 2000/54/EEC of the European Parliament and of the
Council of 18 September 2000 on the protection of workers from risks related
exposure to biological agents at work (seventh individual directive within the meaning
of Article 16(1) of Directive 89/391/EEC.
European Council Decision 96/613/CFSP of 22 October 1996 amending Decision
94/942/CFSP on the joint action adopted by the Council on the basis of Article J.3 of
the Treaty on European Union concerning the control of exports of dual-use goods.
Official Journal No. L 278, 30.10.1996, p. 1
IATA - International Air Transport Association (2010) Dangerous Goods Regulations. 51st
edition. Montreal; Geneva: IATA.
Smith, D. & Desmeth, P. (2007). Access and benefit sharing, a main preoccupation of the
World Federation of Culture Collections. In: UNEP/CBD/WG-ABS/6/INF/3 13
December 2007 Compilation of submissions provided by parties, governments,
indigenous and local communities and stakeholders on concrete options on
substantive items on the agenda of the fifth and sixth meetings of the ad hoc open
ended working group on access and benefit sharing. Canada: UNEP/CBD. p 68-70.
Smith, D. & Rohde, C. (2008) Safety in microbiology. Laboratory Manager Issue 125, 4-6. UK:
Croner.
USEFUL WEBSITES
Legislation and operation
Convention on Biological Diversity |
http://www.cbd.int/ |
| EBRCN Information Resource | http://www.wfcc.info |
| European Commission DGVII – Transport | http://europa.en.int/en/comm/dg07/index.htm |
| Harmonisation of UN documents etc. | www.hazmat.dot.gov/rules |
International Air Transport Association |
|
OECD - Harmonisation Documents |
|
| Chemical programme | http://www.oecd.org/ehs |
| Classification and labelling | http://www.oecd.org/class |
| Chemical testing | http://www.oecd.org/test |
| Currently available test guidelines | http://www.oecd.org/test/testlist |
UN Committee of Experts for the Transport |
www.tc.gc.ca/tdgoods/consult/unlinks_e.htm |
Universal Postal Union |
http://ibis.ib.upu.org |
World Health Organisation |
|
World Federation for Culture Collections |
http://www.wfcc.info |
| Organisations |
Biodiversity and Biological Collections Web
Server
http://muse.bio.cornel .edu/
European Culture Collections’ Organisation
http://www.eccosite.org
MIRCEN Scholarships
http://www.unesco.org/science/life/life1/rcenform.htm
World Federation for Culture Collections
http://www.wfcc.info
World Data Centre for Micro-organisms
http://www.wdcm.nig.ac.jp
Patents
Budapest Treaty on the International
Recognition of the Deposit of
Microorganisms for the Purposes of Patent
Procedure
http://www.wipo.int/treaties/en/registration/budapest/
Code of Practice for IDAs
http://bccm.belspo.be/tbu/ida/index.php
Safety and Standards
Advisory Committee on Dangerous
Pathogens
http://www.doh.gov.uk/bioinfo.htm
Binas Biosafety Site
http://www.un.org/binas
CABRI – Common Access to Biological
http://www.cabri.org
Resources and Information - Guidelines
Cartagena Protocol on Biosafety
http://www.biodiv.org/biosafety/protocol.asp
EC Directive 93/88/EEC on Biological
Agents
http://eur-op.eu.int/opnews/395/en/r3633.html
International Organisation for
Standardisation
http://www.iso.org/iso/en/ISOOnline.frontpage
OECD Best Practice for BRCs
http://www.oecd.org (Search for BRC)
WHO Biosafety Manual
http://www.who.int/csr/resources/publications/biosafety/who_cds_csr_lyo_20034/en/
UK National Culture Col ection (UKNCC)
Quality Management System
http://www.ukncc.co.uk
Taxonomy and Nomenclature
The creation of a new starting date for
prokaryote nomenclature and the
mechanism of valid publication of a name is
defined in the Bacteriological Code
http://www.ncbi.nlm.nih.gov/books/NBK8817/
Publication of the Approved Lists of
Bacterial Names
http://ijs.sgmjournals.org/cgi/reprint/30/1/225
Lists of Bacterial Names is also published in
an amended edition
http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=bacname
Valid publication of names of prokaryotes
according to the rules of nomenclature: past
history and current practice Int J Syst Evol
Microbiol 2006 56: 2715-2720
http://ijs.sgmjournals.org/cgi/content/full/56/11/2715
Matters relating to the deposit and
availability of type strains in collections have
been raised:
Proposals to clarify how type strains are
deposited and made available to the
scientific community for the purpose of
systematic research Int J Syst Evol
Microbiol 2008 58: 1987-1990.
http://ijs.sgmjournals.org/cgi/content/full/58/8/1987
Confirmation of deposit, but confirmation of
what? Int J Syst Evol Microbiol 2008 58:
1785-1787.
http://ijs.sgmjournals.org/cgi/content/full/58/8/1785
A recent review deals with an important
aspect in taxonomy, the characterization of
strains:
Notes on the characterization of prokaryote
strains for taxonomic purposes Int J Syst
Evol Microbiol 2010 60: 249-266
http://ijs.sgmjournals.org/cgi/content/full/60/1/249
See also the ICSP website
http://www.the-icsp.org/
The International Committee on Taxonomy
of Viruses
http://www.ictvonline.org/index.asp?bhcp=1
Virus taxonomy and provides a database of
names
http://www.ncbi.nlm.nih.gov/ICTVdb/
The taxonomy of fungi and yeast is dealt
with by the Botanical Code International
Code of Botanical Nomenclature (VIENNA
CODE)
http://ibot.sav.sk/icbn/main.htm
Index Fungorum
http://www.indexfungorum.org/
MycoBank
http://www.mycobank.org/
The Botanical Code also covers algae
(including
cyanobacteria/cyanophytes) and includes
protozoa considered to be botanical taxa.
This is governed by the IAPT - International
Association for Plant Taxonomy
http://www.botanik.univie.ac.at/iapt/index_layer.php
The International Code of Zoological
http://www.iczn.org/iczn/index.jsp
Nomenclature also covers protozoa
considered to be zoological taxa
International Commission on Zoological
Nomenclature
http://www.iczn.org/
ZooBank
http://www.zoobank.org/
USEFUL ADDRESSES
WDCM - World Data Centre for Microorganisms Contacts: Dr. Juncai Ma, Institute of Microbiology, Chinese Academy of Sciences. NO.1 Beichen West Road, Chaoyang District, Beijing 100101, China . Tel: +86-10-64807422. Fax:+86-10-64807426. email:ma@im.ac.cn
WFCC - World Federation for Culture Collections Contacts: Philippe Desmeth, BCCM, Federal Public Planning Service – Science Policy avenue Louise, 231 1050 Brussels,Belgium. Secretary: Ms Anne Depauw. email:depa@belspo.be
GBRCN – Global Biological Resource Centre Network demonstration project Secretariat
Julius Kühn-Institut (JKI), Bundesforschungsinstitut für Kulturpflanzen (Federal Research
Centre for Cultivated Plants), Institute for Crop and Soils Science, Bundesallee 50,
D-38116 Braunschweig
Tel: +49 531 596 2298
http://www.gbrcn.org
ECCO – European Culture Collections' Organisation
http://www.eccosite.org/
ACM - Asian Collections of Microorganisms
c/o Dr. Ken Ichiro Suzuki, NITE Biological Resource Center, National Institute of Technology and Evaluation, 2-5-8 Kazusakamatari, Kisarazu-shi, Chiba, 292-0818 Japan
Copyright 2010 World Federation for Culture Collections All rights reserved.
 |
 |
 |
| English Version (Download) | Korean Version (Download) | Spanish Version (Download) |
- Chinese Version (Download)
- Japanese Version (Download)
- German Version (Download)

